Nuclear power plants
by Chris Woodford. Last updated: March 16, 2025.
Atomic energy has had a mixed history in the half-century or so since the world's first commercial nuclear power plant opened at Calder Hall (now Sellafield) in Cumbria, England in 1956. Huge amounts of world energy have been produced from atoms ever since, but amid enormous controversy. Some people believe nuclear power is a vital way to tackle climate change; others insist it is dirty, dangerous, uneconomic, and unnecessary. Either way, it helps if you understand what nuclear energy is and how it works—so let's forget the politics for a moment and take a closer look at the science.
Photo: Nuclear power plants need large supplies of cooling water, which is why they're often built in coastal areas. Palo Verde Nuclear Generating Station near Phoenix, Arizona uses these spray ponds as a form of backup cooling. Credit: Photographs in the Carol M. Highsmith Archive, Library of Congress, Prints and Photographs Division.
Sponsored links
Contents
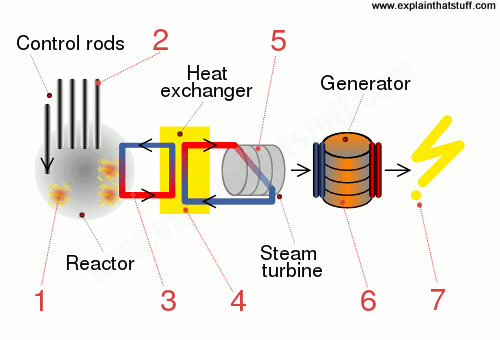
What is atomic energy?
It's not immediately obvious but tall buildings store energy—potential energy. You have to work hard to lift bricks and other building materials up off the ground into the right position and, as long as they remain where you put them, they can store that energy indefinitely. But a tall, unstable building is bound to collapse sooner or later and, when it does so, the materials from which it was built come crashing back down to the ground, releasing their stored potential energy as heat, sound, and kinetic energy (the bricks could fall on your head!).
Atoms (the building blocks of matter) are much the same. Some large atoms are very stable and quite happy to stay as they are pretty much forever. But other atoms exist in unstable forms called radioactive isotopes. They're the atomic equivalents of wobbly old buildings: sooner or later, they're bound to fall apart, splitting into bits like a large building tumbling to the ground and releasing energy on the way. When large atoms split into one or more smaller atoms, giving off other particles and energy in the process, we call it nuclear fission. That's because the central part of the atom (the nucleus) is what breaks up and fission is another word for splitting apart. Nuclear fission can happen spontaneously, in which we case we call it radioactive decay (the conversion of unstable, radioactive isotopes into stable atoms that aren't radioactive). It can also be made to happen on demand—which is how we get energy out of atoms in nuclear power plants. That type of fission is called a nuclear reaction.

Photo: Carefully controlled: Before it was closed in the 1970s, NASA's scientific nuclear reactor at Plum Brook Station in Sandusky Ohio was used for developing materials for the space program. The site has now been renamed NASA Armstrong (for astronaut Neil Armstrong) and does other kinds of cutting-edge space research. Picture courtesy of NASA Glenn Research Center (NASA-GRC) and Internet Archive.
How much energy can one atom make?
A surprisingly large amount! That was what physicist Albert Einstein meant when he wrote out this simple and now famous equation:
E = mc2
If E is energy, m is mass (the scientific word for the ordinary stuff around us), and c is the speed of light, Einstein's equation says that you can turn a tiny amount of mass into a huge amount of energy. How come? Looking at the math, c is a really huge number (300,000,000) so c2 is even bigger: 90,000,000,000,000,000. That's how many joules (the standard measurement of energy) you'd get from a kilogram of mass. In theory, if you could turn about seven billion hydrogen atoms completely to energy, you'd get about one joule (that's about as much energy as a 10-watt lightbulb consumes in a tenth of a second). Remember, though, these are just ballpark, guesstimate numbers. The only point we really need to note is this: since there are billions and billions of atoms in even a tiny spec of matter, it should be possible to make lots of energy from not very much at all. That's the basic idea behind nuclear power.

Photo: Albert Einstein—godfather of nuclear energy. Photo courtesy of US Library of Congress.
In practice, nuclear power plants don't work by obliterating atoms completely; instead, they split very large atoms into smaller, more tightly bound, more stable atoms. That releases energy in the process—energy we can harness. According to a basic rule of physics called the law of conservation of energy, the energy released in a nuclear fission reaction is equal to the total mass of the original atom (and all the energy holding it together) minus the total mass of the atoms it splits into (and all the energy holding them together). For a more detailed explanation of why nuclear reactions release energy, and how much they can release, see the article binding energy on Hyperphysics.

Artwork: Atoms are made of protons (red), neutrons (blue), electrons (green), and energy binding them together (yellow). By splitting large unstable atoms into smaller and more stable ones, we can release some of this "binding energy." That's where nuclear power plants get their energy from.
What is a chain reaction?
What if you could make lots of atoms split up one after another? In theory, you could get them to release a huge amount of energy. If breaking up billions of atoms sounds like a real bore (like breaking billions of eggs to make an omelet), there's one more handy thing that helps: some radioactive isotopes will go on splitting themselves automatically in what's called a chain reaction, producing power for pretty much as long as you want.
Suppose you take a really heavy atom—a stable kind of uranium called uranium-235. Each of its atoms has a nucleus with 92 protons and 143 neutrons. Fire a neutron at uranium-235 and you turn it into uranium-236: an unstable version of the same atom (a radioactive isotope of uranium) with 92 protons and 144 neutrons (remember that you fired an extra one in). Uranium-236 is too unstable to hang around for long so it splits apart into two much smaller atoms, barium and krypton, releasing quite a lot of energy and firing off three spare neutrons at the same time.
Now the brilliant thing is that the spare neutrons can crash into other uranium-235 atoms, making them split apart too. And when each of those atoms splits, it too will produce spare neutrons. So a single fission of a single uranium-235 atom rapidly becomes a chain reaction—a runaway, nuclear avalanche that releases a huge amount of energy in the form of heat.

Photo: Chain reaction! Fire a neutron (1) at a large uranium-235 atom (2). You make an even larger, unstable radioactive isotope of uranium, uranium-236, that promptly splits into two smaller and more stable atoms krypton and barium (3). In the process, heat energy is released and there are three spare neutrons left over (4). The neutrons can go on to react with more uranium-235 atoms (5) in a hugely energetic chain reaction. Other fission reactors are possible when a neutron hits uranium-235, producing either two or four spare neutrons. That's why (confusingly) you'll sometimes read in books that uranium-235 fission produces "two or three" spare neutrons (and an average of 2.47) per reaction.
What's the difference between a nuclear power plant and a nuclear bomb?
In a nuclear bomb, the chain reaction isn't controlled, and that's what makes nuclear weapons so terrifyingly destructive. The entire chain reaction happens in a fraction of a second, with one splitting atom producing two, four, eight, sixteen, and so on, releasing a massive amount of energy in the blink of an eye. In nuclear power plants, the chain reactions are very carefully controlled so they proceed at a relatively slow rate, just enough to sustain themselves, releasing energy very steadily over a period of many years or decades. There is no runaway, uncontrolled chain reaction in a nuclear power plant.