Catalytic converters
by Chris Woodford. Last updated: February 18, 2022.
Blackened buildings and choking streets—if that's your experience when you open the front door in the morning, you probably live in a big city like Los Angeles, London, Paris, or Beijing. Cars, buses, and trucks have been a great gift to the world, because they help us move ourselves (and the things we need) quickly and efficiently. But their engine pollution spoils the places where we live and harms our health. Fortunately, most vehicles are now fitted with pollution-reducing units called catalytic converters (sometimes known as "cats" or "cat-cons"), which turn the harmful chemicals in vehicle exhausts into harmless gases such as steam. Let's take a closer look at these brilliant gadgets and how they work!
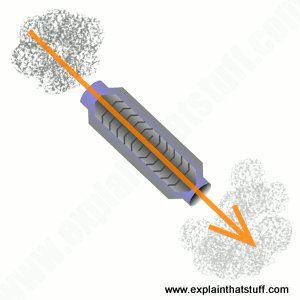
Artwork: The basic concept of a catalytic converter: sitting between your car's engine and tailpipe, it takes in dirty air and removes a significant amount of pollution from it using chemical catalysts.
Sponsored links
Contents
Why engines make pollution
Car engines run on gasoline or diesel, which are made from petroleum. Most of our petroleum is formed when the remains of tiny sea creatures rot down, heat up, and get squeezed by layers of sea-bed rocks. Petroleum is made up of hydrocarbons (molecules built from carbon and hydrogen atoms) because living organisms are mostly made from those atoms too.
In theory, if you burn any kind of hydrocarbon fuel with oxygen from the air, you release a lot of energy and make nothing but carbon dioxide and water, which are clean and relatively harmless. In practice, though, gasoline is a mixture of about 150 different chemicals, not just hydrocarbons but additives too, and it doesn't burn as cleanly as we'd like. That means you generally get some air pollution as a byproduct. The pollutant gases made by car engines include a poisonous gas called carbon monoxide, as well as VOCs (volatile organic compounds) and nitrogen oxides that cause smog (the sort of choking, cloudy vehicle pollution we all know and hate).

Photo: The columns of the Parthenon in Athens, Greece have been blackened by vehicle pollution. Athens is one of the world's most traffic-polluted cities. Photo by Michael M. Reddy courtesy of U.S. Geological Survey.
What is a catalytic converter?
Pollutant gases are made of harmful molecules, but those molecules are made from relatively harmless atoms. So if we could find a way of splitting up the molecules after they leave a car's engine and before they get pumped out into the air, we could crack the problem of pollution—or some of it, anyway. That's the job that a catalytic converter does.
These gadgets are much simpler than they sound. A catalyst is simply a chemical that makes a chemical reaction go faster without itself changing in the process. It's a bit like an athletics coach who stands by the side of the track and shouts at the runners to go faster. The coach doesn't run anywhere; he just stands there, waves his arms about, and makes the runners speed up. In a catalytic converter, the catalyst's job is to speed up the removal of pollution. The catalyst is made from platinum or a similar, platinum-like metal such as palladium or rhodium.
A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. One of them (the converter's "input") is connected to the engine and brings in hot, polluted fumes from the engine's cylinders (where the fuel burns and produces power). The second pipe (the converter's "output") is connected to the tailpipe (exhaust). As the gases from the engine fumes blow over the catalyst, chemical reactions take place on its surface, breaking apart the pollutant gases and converting them into other gases that are safe enough to blow harmlessly out into the air.

Photo: An experimental new catalytic converter designed to reduce the polluting effects of unburned fuel, nitrogen oxides and particulates. Picture courtesy of Oak Ridge National Laboratory published on Flickr under a Creative Commons (CC BY 2.0) licence.
One very important thing to note about catalytic converters is that they require you to use unleaded fuel, because the lead in conventional fuel "poisons" the catalyst and prevents it from taking up the pollutants in exhaust gases.
What happens inside the converter?
Inside the converter, the gases flow through a dense honeycomb structure made from a ceramic and coated with the catalysts. The honeycomb structure means the gases touch a bigger area of catalyst at once, so they are converted more quickly and efficiently.
Typically, there are two different catalysts in a catalytic converter:
- One of them tackles nitrogen oxide pollution using a chemical process called reduction (removing oxygen). This breaks up nitrogen oxides into nitrogen and oxygen gases (which are harmless, because they already exist in the air around us).
- The other catalyst works by an opposite chemical process called oxidation (adding oxygen) and turns carbon monoxide into carbon dioxide. Another oxidation reaction turns unburned hydrocarbons in the exhaust into carbon dioxide and water.
In effect, three different chemical reactions are going on at the same time. That's why we talk about three-way catalytic converters. (Some, less-effective converters carry out only the second two (oxidation) reactions, so they're called two-way catalytic converters.) After the catalyst has done its job, what emerges from the exhaust is mostly nitrogen, oxygen, carbon dioxide, and water (in the form of steam).

Photo: Engineers are constantly trying to improve the performance of catalytic converters, for example, by developing catalysts that work more effectively at lower temperatures. This is an example of a low-temperature oxidation catalyst made from tin oxide and platinum. Photo by CPL Bryant V courtesy of NASA Langley Research Center (NASA-LaRC) and Internet Archive.
How effective are catalytic converters?
Cats make a big difference to emissions, with three-way converters giving a significant extra benefit over two-way converters:
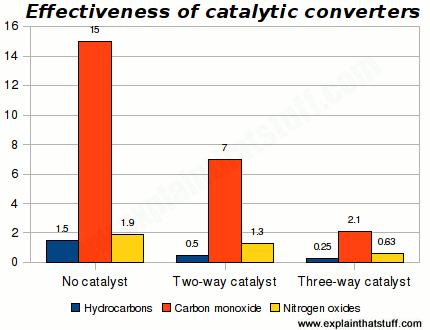
Chart: Effectiveness of catalytic converters. Figures show pollutants in grams per kilometer at 80,000 kilometers. Chart drawn by Explain that Stuff.com using data for light-duty gasoline fueled vehicles from US EPA (1990), quoted in table 3.2 (page 75) of Air Pollution from Motor Vehicles: Standards and Technologies for Controlling Emissions, Faiz et al, World Bank, 1996.
Catalytic converters are mainly designed to reduce immediate, local air pollution—dirty air where you're driving—and this chart certainly seems to suggest that they're effective. Even so, people sometimes question whether they're really as green as they seem. It's important to remember that they reduce emissions rather than eliminate them completely.
One problem is that they only really work at high temperatures (over 300°C/600°F or so), when the engine has had chance to warm up. Early types of catalytic converters typically took about 10–15 minutes to warm up, so they were completely ineffective for the first few kilometers/miles of a journey (or any part of a very short journey). Modern converters warm up in only 2–3 minutes; even so, significant emissions can still occur during this time.

Chart: Catalytic converters only become efficient at high operating temperatures. This chart shows the efficiency of a typical device at converting carbon monoxide at a range of different temperatures. Nitrogen oxides are converted with slightly higher efficiency and hydrocarbons with slightly less efficiency. At high temperatures, carbon monoxide is converted with the least efficiency of the three.
Another issue is whether they increase greenhouse gas emissions. We think of carbon dioxide as a safe gas, because it's not toxic in everyday concentrations. Nevertheless, it isn't entirely harmless, because we now know it's the major cause of global warming and climate change. Some people believe catalytic converters make climate change worse because they turn carbon monoxide into carbon dioxide. In fact, the carbon monoxide your car produces would eventually turn into carbon dioxide in the atmosphere all by itself, so a catalytic converter makes no difference on that score: it simply reduces the carbon monoxide a car pumps into the street as it drives along, improving the local air quality.
But when it comes to climate change, auto engineers and environmentalists have long pointed out another serious issue. Although cats turn most nitrogen oxides into nitrogen and oxygen, they also produce small amounts of nitrous oxide (N2O) in the process, a greenhouse gas that's over 300 times more potent than carbon dioxide. The trouble is that with so many vehicles on the road, even small amounts of nitrous oxide add up to a major problem. Back in 2000, the Intergovernmental Panel on Climate Change noted: "The introduction of catalytic converters as a pollution control measure in the majority of industrialized countries is resulting in a substantial increase in N2O emissions from gasoline vehicles." Fortunately, newer catalytic converters produce dramatically less nitrous oxide than older ones. Even so, while catalytic converters have certainly helped us to tackle short-term air pollution, there are concerns that, when it comes to long-term climate change, they could be making matters worse.






