Vacuum flasks
by Chris Woodford. Last updated: August 8, 2023.
Humans can be pretty contrary at the best of times. When it's cold, we want to warm up; when it's hot, we want to cool down. That's because we're warm-blooded creatures who need to keep our body temperatures more or less constant, at around 37°C (98.6°F), just to survive. Vacuum flasks are a bit like people in this respect: they like to keep things at steady temperatures. If you put hot drinks in them, they keep them hot; if you put cold drinks in them, they keep them cool. They're simple, neat, and effective—but how exactly do they work?
Photo: A typical Thermos® vacuum flask. Vacuum flasks are widely known as "Thermos" flasks for the German company, founded by Reinhold Burger, that commercialized the technology in 1904.
Sponsored links
Contents
How heat travels
Before we can understand why flasks are so fantastic, we need to understand a bit more about how heat travels.
Heat is a kind of energy that moves around our world in three different ways called conduction, convection, and radiation. If you touch something hot, heat flows straight into your body because there's a direct connection between you and the hot object. Heat conduction happens only when things touch.
Convection, on the other hand, can happen without the need for direct contact. If you switch on a fan heater, it blows hot air through a grille into your room. Hot air is less dense (lighter, effectively) than cold air so it rises upwards. As hot air starts to climb up from a fan heater, it has to push colder air out of its way. So the cooler air near the ceiling of your room moves back toward the floor to get out of the way. Pretty soon, there's a kind of invisible conveyor belt of warming, rising air and cooling falling air and this gradually warms up the room. When heat moves in this way, using a moving liquid or gas to travel from one place to another, we call it convection. Heating soup in a saucepan is another way of using convection.
Radiation is slightly different again from conduction and convection. When objects are hot, they give off light. That's why camp fires glow red, orange, and yellow. This happens because the atoms in hot objects become "excited" and unstable when they gain extra heat energy from the fire. Since they're unstable, the atoms quickly return to their normal state—and give off the energy they had as light. (Read more about how and why this happens in our longer article about light.) Sometimes we can see the light that atoms produce and sometimes not. If the light they produce is just a bit too red for our eyes to see, it's called infrared radiation and, rather than seeing it, we feel it as heat. You can feel the infrared given off by hot objects even if you're not touching them (so there's no conduction) and there's no air or liquid present to carry heat either (so there's no convection). Radiation explains why we can feel heat "beaming" from hot things nearby even when we're not touching them (conduction) or being warmed by the hot air they're generating (convection).
You can read much more about heat energy in our main article on heat.
Why your coffee goes cold
Suppose you've just made a hot pot of coffee. You'll be well aware that you need to drink it quickly before it goes cold—but why does it go cold? Boiling water has a temperature of 100°C (212°F), while room temperature is more likely to be 15-20°C (60-70°F), depending on the weather and whether you have your heating on. Since the water in your drink is so much hotter than the room, heat flows rapidly from the coffee pot into the surroundings. Some heat will be lost by conduction: because your coffee pot is standing on a table or worktop, heat will flow directly downward and disappear that way. The air directly above and all around the pot will be warmed by it and start moving around, so more heat will be lost by convection. And some heat will also be lost by radiation.
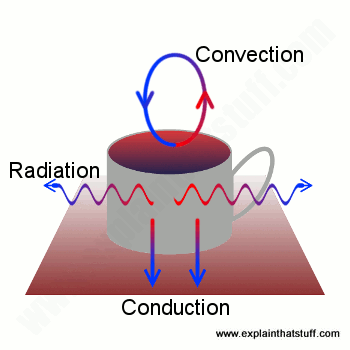
Artwork: Your coffee cools through a mixture of conduction, convection, and radiation.
Together, conduction, convection, and radiation will turn piping hot coffee into something cold, miserable and yucky in less than an hour. If you want your coffee to stay hot, you need to stop conduction, convection, and radiation from happening. And you can do that by putting your coffee into a vacuum flask.
How vacuum flasks work
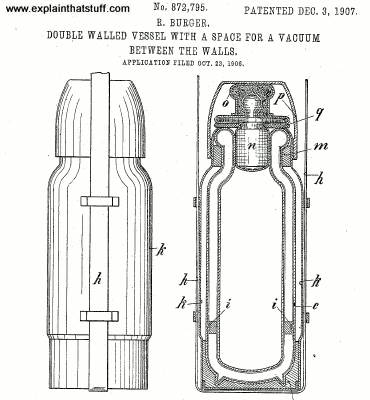
A vacuum flask is a bit like a super-insulated jug. Most versions have an inner chamber and an outer plastic or metal case separated by two layers of glass with a vacuum in between. The glass is usually lined with a reflective metal layer. Unbreakable flasks do away with the glass. Instead, they have two layers of stainless steel with a vacuum and a reflecting layer in between them. There's also a tight, screw-down stopper on the top.

Photo: With the stopper removed, you can clearly see the reflective glass inside this Thermos flask. Two more things to note as well: first, the neck is quite narrow (so helping to reduce heat losses) and the liquid-carrying capacity of the flask is much less than you'd expect from its overall size. That's because quite a lot of space is taken up by the insulating air gap between the inner container and the outer plastic wall.
These few, simple features prevent virtually all heat transfer by either conduction, convection, or radiation. The vacuum prevents conduction. The tight stopper prevents air from entering or leaving the flask, so convection isn't possible either. What about radiation? When infrared radiation tries to leave the hot liquid, the reflective lining of the inner chamber reflects it straight back in again. There's virtually no way heat can escape from a vacuum flask and a hot drink stored inside will stay steaming hot for several hours.
Flasks also work for cold drinks. If heat can't escape from a vacuum flask, it follows that heat can't penetrate into a flask from outside either. The sealed stopper stops heat getting in by convection; the vacuum stops conduction, and the metal lining between the outer case and the inner chamber stops heat radiating in either.
Whether you like your coffee piping hot or icy cold, vacuum flasks are an absolutely brilliant way to keep your drinks just the way you want. Some heat still escapes (or gets in) eventually, mostly through the stopper, but flasks like this are still a vast improvement on virtually every other kind of insulated drinks container.